|
April 7, 1976
- Venture capitalist Robert A. Swanson, biochemist Dr.
Herbert W. Boyer (pioneered recombinant DNA technology) founded
biotechnology company, Genentech in South San Francisco in
one rented building and two staff members;
1977 - produced first human protein (somatostatin)
in microorganism (E. coli bacteria); 1978 - cloned
human insulin; 1979 - cloned human growth hormone;
1980 - went public; 1982 - marketed First
recombinant DNA drug, human insulin (licensed to Eli Lilly and
Company); 1985 - received approval from U.S. Food
and Drug Administration (FDA) to market first product, Protropin®
(somatrem for injection) growth hormone for children with growth
hormone deficiency (first recombinant pharmaceutical product
manufactured, marketed by biotechnology company); 1990
- majority interest
(56%) acquired by Roche Holdings Ltd. (Basel, Switzerland) in
$2.1 billion deal; total assets of $1.1 billion;
1995 - extended for four years
Roche's option to purchase outstanding redeemable common stock
of company at predetermined price; 1997 - launched
a service for patients, their physicians called SPOC (Single
Point of Contact) to provide customer-focused reimbursement
assistance (renamed Genentech Access Solutions in 2008);
1998 - dedicated $250 million manufacturing facility in
Vacaville, CA (received FDA licensure as multi-product facility
in 2000); 1999 - Roche Holdings, Inc. exercised
its option, Genentech redeemed all of outstanding special common
shares not owned by Roche; announced its intent to publicly sell
up to 19% of Genentech shares, continue Genentech as publicly
traded company with independent directors; July 20, 1999
- went public, considered largest public offering in history of
U.S. health care industry (NYSE trading symbol, DNA);
October 20, 1999 - Roche conducted secondary offering of
20 million Genentech shares, largest secondary offering in U.S.
history; 2008 - FORTUNE magazine named Genentech
(#5) one of "100 Best Companies to Work For" (tenth consecutive
year);
March 26, 2009 -
44% interest (balance of outstanding shares) acquired by Roche
for $47 billion; 7th-largest pharmaceutical company (market
share), annual sales of $17 billion, 17,500 employees (in U.S.
segment).
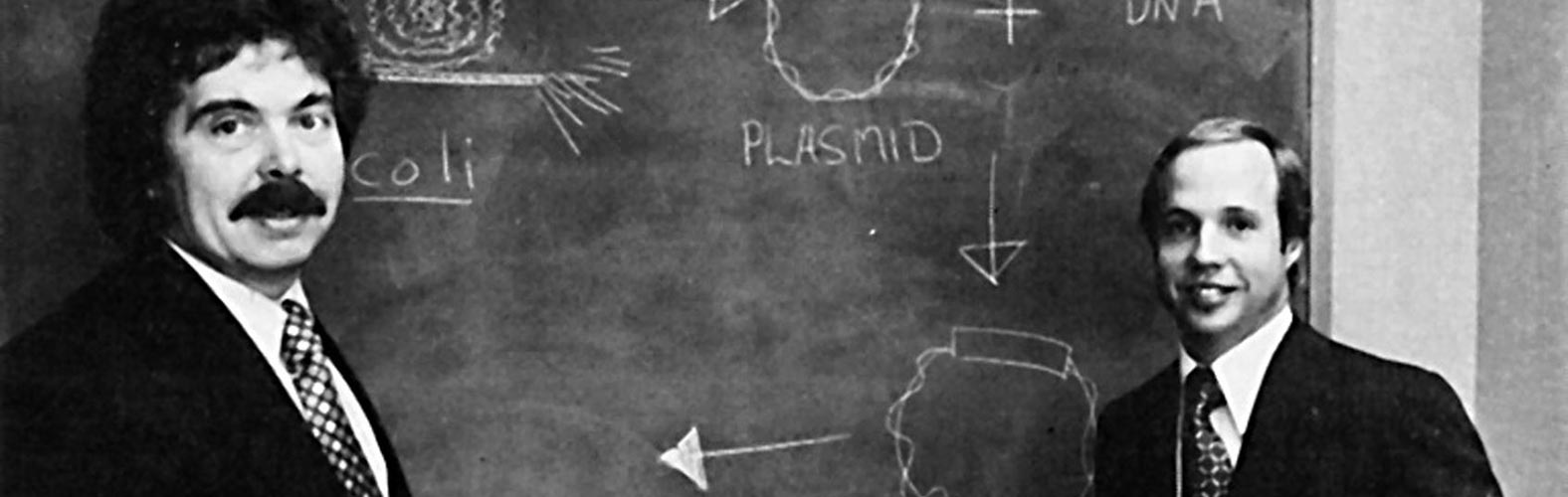 Dr. Herbert W.
Boyer, Robert A. Swanson
- Genentech
(http://www.gene.com/assets/frontend/img/content/about-us/leadership/inline_founders.jpg)
Dr. Herbert W.
Boyer, Robert A. Swanson
- Genentech
(http://www.gene.com/assets/frontend/img/content/about-us/leadership/inline_founders.jpg)
June 16, 1980
- Supreme Court ruled in Diamond v. Chakrabarty that living,
manmade microorganisms which are products of "human
ingenuity and research" and not "nature's handiwork" are
patentable; microbiologist, Ananda Chakrabarty, distinguished
professor of microbiology and immunology at the University of
Illinois College of Medicine, appealed had rejection of
his 1972 patent application for human-made, genetically
engineered bacterium capable of breaking down crude oil, which
no naturally occurring bacteria could do.
March 31, 1981
- Ananda M. Chakrabarty, of Latham, NY, received a patent for
"Microorganisms Having Multiple Compatible Degradative
Energy-Generating Plasmids and Preparation Thereof"; single cell
genetically engineered life form; assigned to General Electric
Company.
June 18, 1981 -
First genetically engineered vaccine announced: first
effective subunit vaccine for any animal or human disease using
gene splicing; designed to prevent hoof and mouth disease (FMD);
1980 - U.S. Dept of Agriculture scientists turned
to recombinant DNA technology, collaborated with scientists from
Genentech, a private company; inserted a bioengineered plasmid
containing the gene for VP3 into Escherichia coli bacteria which
grew obeying orders from the guest DNA, mass-producing the VP3
proteins for the vaccine.
April 16, 1987 -
U.S. government authorized patents on genetically engineered
processes, first nation in world to allow such patent
applications.
April 12, 1988 -
Geneticists Philip Leder, of Chestnut Hill, MA, and Timothy A.
Stewart, of San Francisco, CA, received a patent for "Transgenic
Non-Human Mammals"; assigned to the President and Fellows of
Harvard University; designed to be highly susceptible to breast
cancer; designated as "oncomice," intended for use in testing
anticancer therapies with more efficiency and accurate results.
August 1991 -
Calgene Inc. (Davis, CA) submitted first commercially grown
genetically engineered food to U.S. Food and Drug Administration
for approval; first time FDA had evaluated whole food produced
by biotechnology (vs. cross-breeding); May 18, 1994
- Food and Drug Administration approved Flavr Savr, new tomato
developed through biotechnology, as safe as tomatoes bred by
conventional means .
April 23, 2007
- AstraZeneca agreed to pay $15.6 billion for MedImmune, maker
of FluMist nasal spray flu vaccine = largest acquisition of
biotechnology company in history; reflected growth by
acquisition strategy as patent protection lapsed for
pharmaceutical companies' existing drug products.
May 2008 -
Ernst & Young's annual
financial report on biotech industry cited 2007 financing from
all sources topped $21.3 billion for U.S. biotech companies;
$5.5 billion contributed by venture capital firms beat record
set in 2000 (Human Genome Project); of 386 publicly traded U.S.
biotechnology companies, 49% have more than two years of cash on
hand (27% of those) have more than five years of cash; total
potential value of mergers, acquisitions drug development
alliances was nearly $60 billion in United States, surpassed
levels in all prior years; number of approvals for new drugs
dropped to lowest level in two decades (FDA faced resource
constraints, lawmakers pressed for more-stringent safety
reviews); market capitalization of San Francisco Bay Area's 77
public companies was $148.6 billion (40% of total market value
of U.S. biotech companies) on revenue of $22.1 billion (34% of
U.S. total for the sector); Boston area's 62 public companies
had market capitalization of $65.1 billion (17.6% of U.S.
biotech market capitalization).
(Amgen), Gordon Binder (2008).
Science Lessons: What the Business of Biotech Taught Me About
Management. (Boston, MA: Harvard Business School Press,
292 p.). Former Chief Financial Officer, Chief Executive
Officer, Chairman of Amgen (1982 -2000). Biotechnology
industries -- United States -- Management; Chief executive
officers -- United States -- Biography; Biotechnology --
economics -- Personal Narratives; Biotechnology -- history --
Personal Narratives; History, 20th Century -- Personal
Narratives; Industry -- Personal Narratives.
Amgen's climb to success, highs
and lows in race to develop blockbuster drugs; 1989 - launch of
Epogen, Neupogen followed; managing creative employees,
navigating IPO process, protecting intellectual property.
(CellPro), Rick Murdock with David Fisher
(2000).
Patient Number One: A True Story of How One CEO Took on Cancer
and Big Business in the Fight of His Life. (New York,
NY: Crown, 308 p.). Murdock, Rick--Health;
Lymphomas--Patients--Washington--Seattle--Biography; Cell
separation; Biotechnology industries--Washington--Seattle.
(Cetus Corporation), Paul Rabinow (1996).
Making PCR: A Story of Biotechnology. (Chicago, IL:
University of Chicago Press, 190 p.). Polymerase chain reaction
-- History.
(Genentech), Maureen D. McKelvey (1996).
Evolutionary Innovations: The Business of Biotechnology.
(New York, NY: Oxford University Press, 319 p.). Genentech,
Inc.; KabiVitrum Sverige AB; - Genetic engineering;
Biotechnology industries--United States; Biotechnology
industries--Sweden; Recombinant human insulin; Recombinant human
somatotropin.
(ImClone Systems), Alex Prud'homme (2004).
The Cell Game: Sam Waksal's Fast Money and False Promises--and
the Fate of ImClone's Cancer Drug. (New York, NY:
HarperCollins, 288 p.). Waksal, Samuel David; ImClone Systems
Incorporated--History; Businessmen--United States--Biography;
Antineoplastic agents industry--Corrupt practices--United
States; Monoclonal antibodies--Research--United States--History;
Cancer--Chemotherapy; Drugs--United States--Testing; Insider
trading in securities--United States.
(Monsanto), Peter Pringle (2003).
Food, Inc.: Mendel to Monsanto--The Promises and Perils of the
Biotech Harvest. (New York, NY: Simon & Schuster, 239
p.). Agricultural biotechnology; Genetically modified foods;
Food--Biotechnology.
Robert Bud (1993).
The Uses of Life: A History of Biotechnology. (New York,
NY: Cambridge University Press, 299 p.). Biotechnology--History.
How modern biotechnology
grew out of this century's hopes for new relationship between
biology, engineering.
Claire Hope Cummings (2008).
Uncertain Peril: Genetic Engineering and the Future of Seeds.
(Boston, MA: Beacon Press, 240 p.). Agricultural
biotechnology--Political aspects.; Transgenic plants--Economic
aspects; Transgenic plants--Risk assessment; Consumer
protection--Citizen participation; Seeds--Biotechnology.
Interdependence between plants, people amidst privatization of Earth's seed stock.
Janet Hope (2007).
Biobazaar: The Open Source Revolution and Biotechnology.
(Cambridge, MA: Harvard University Press,, 448 p.). Member of
the Australian National University's Center for Governance of
Knowledge and Development. Biotechnology--Patents; Technological
innovations--Patents; Patent licenses; Biotechnology--Economic
aspects; Technological innovations--Economic aspects.
Appeal of open source approach
lies in its safeguarding of community access to proprietary
tools without discouraging valuable commercial participation;
detailed picture of "open source biotechnology" as desirable,
broadly feasible.
Ed. Robert W. Kolb (2007).
The Ethics of Genetic Commerce. (Malden, MA: Blackwell
Pub., 240 p.). Frank W. Considine Chair in Applied Ethics
(Loyola University Chicago). Genetic engineering industry;
Genetic engineering--Moral and ethical aspects; Genetic
screening; Genetically modified foods. Moral, ethical concerns derived
from increasing knowledge of genetics, variety of its commercial
applications (genetic screening, use of individual’s genetic
information, rise of genetically modified foods, patenting,
pharmaceutical mergers and monopolization, implications of
genetic testing on non-human mammals).
Sharon McAuliffe and Kathleen McAuliffe
(1981).
Life for Sale. (New York, NY: Coward, McCann &
Geoghegan, 243 p.). Genetic engineering--Economic aspects;
Genetic engineering--Social aspects; Recombinant DNA--Economic
aspects; Recombinant DNA--Social aspects.
Chris Meyer and Stan Davis (2003).
It's Alive: The Coming Convergence of Information, Biology, and
Business. (New York, NY: Crown, 275 p.). Director,
Research Fellow, respectively (Cap Gemini Ernst & Young's Center
for Business Innovation). Information technology--Economic
aspects; Life cycles (Biology); Business cycles.
Luigi Orsenigo (1989).
The Emergence of Biotechnology: Institutions and Markets in
Industrial Innovation. (New York, NY: St. Martin's
Press, 230 p.). Biotechnology industries--History.
Gary P. Pisano (2006).
The Science Business: The Promise, the Reality, and the Future
of Biotech. (Boston, MA: Harvard Business School Press,
237 p.). Harry E. Figgie, Jr. Professor of Business
Administration (Harvard Business School). Biotechnology
industries--History. Science-based business poses 3 unique challenges: 1) how to
finance highly risky investments under profound uncertainty and
long time horizons for R&D, 2) how to learn rapidly enough to
keep pace with advances in drug science knowledge, and 3) how to
integrate capabilities across a broad spectrum of scientific and
technological knowledge bases.
Cynthia Robbins-Roth (2001).
From Alchemy to IPO: The Business of Biotechnology.
(Cambridge, MA: Perseus Pub., 253 p.). Biotechnology -- History;
Biotechnology industries.
Edward J. Sylvester and Lynn C. Klotz (1987).
The Gene Age: Genetic Engineering and the Next Industrial
Revolution (New York, NY: Scribner, 239 p. [rev. ed.]).
Genetic engineering.
Robert Teitelman (1989).
Gene Dreams: Wall Street, Academia, and the Rise of
Biotechnology. (New York, NY: Basic Books, 237 p.).
Genetic engineering industry; Biotechnology industries.
Eric J. Vettel (2006).
Biotech: The Countercultural Origins of an Industry.
(Philadelphia, PA: University of Pennsylvania Press, 273 p.).
Bancroft Postdoctoral Fellow in United States History
(University of California, Berkeley), Founding Executive
Director of the Woodrow Wilson Presidential Library in Staunton
Virginia. Biotechnology industries --History.
Story behind genetic engineering,
recombinant DNA, cloning, stem-cell research - practical
application of biological knowledge supported by private
investors expecting profitable returns eclipsed basic research
supported by government agencies.
Junfu Zhang and Nikesh Patel (2005).
The Dynamics of California’s Biotechnology Industry.
(San Francisco, CA: Public Policy Institute of California, 139
p.). Biotechnology industries--California; Venture
capital--California. State
accounts for 47% of national R&D spending on
biotechnology, generates 53% of nation's biotech
revenues.
|

